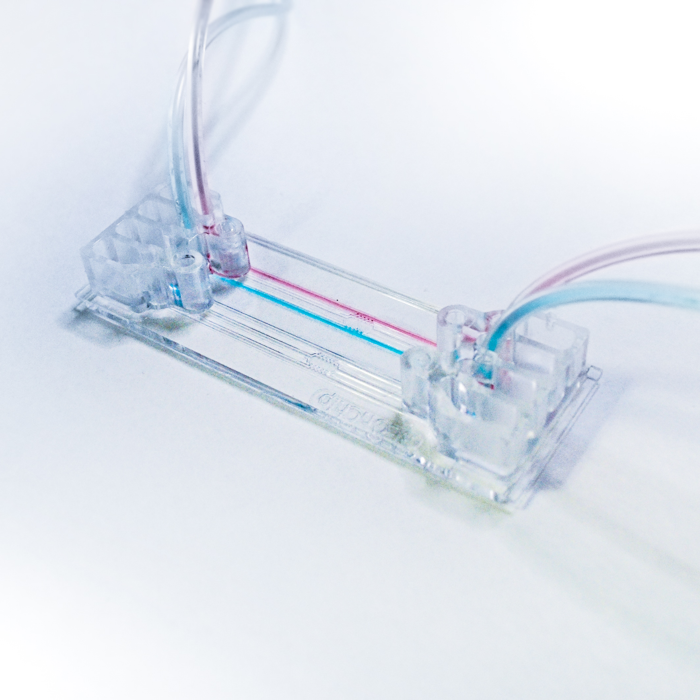
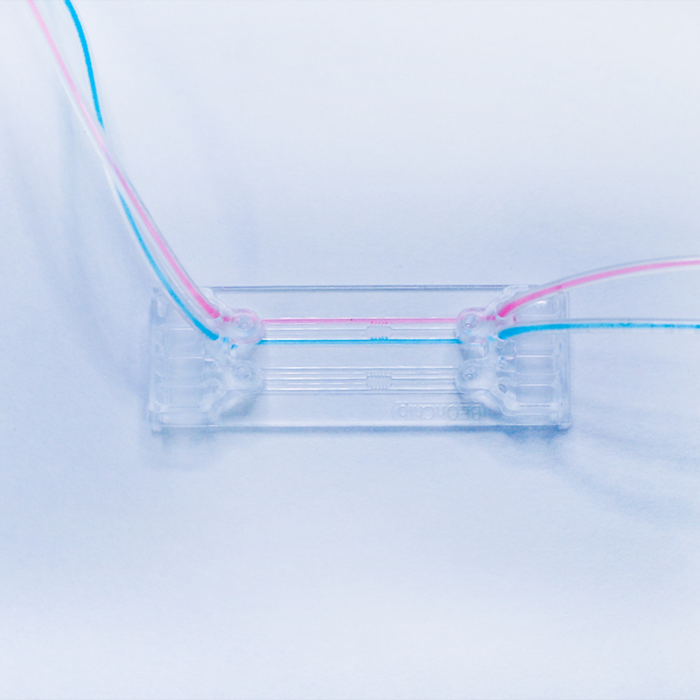
BE-GRANDIENT Microfluidic Chip
BE-GRADIENT is a versatile microfluidic device for cell culture under bionic conditions.It can be used for cell culture under chemical gradients.Because of the optical transparency of the polymers used, it is possible to monitor experiments using phase contrast microscopes, fluorescence microscopes and confocal microscopes.
▲Technical Parameters


Description
I. Overview
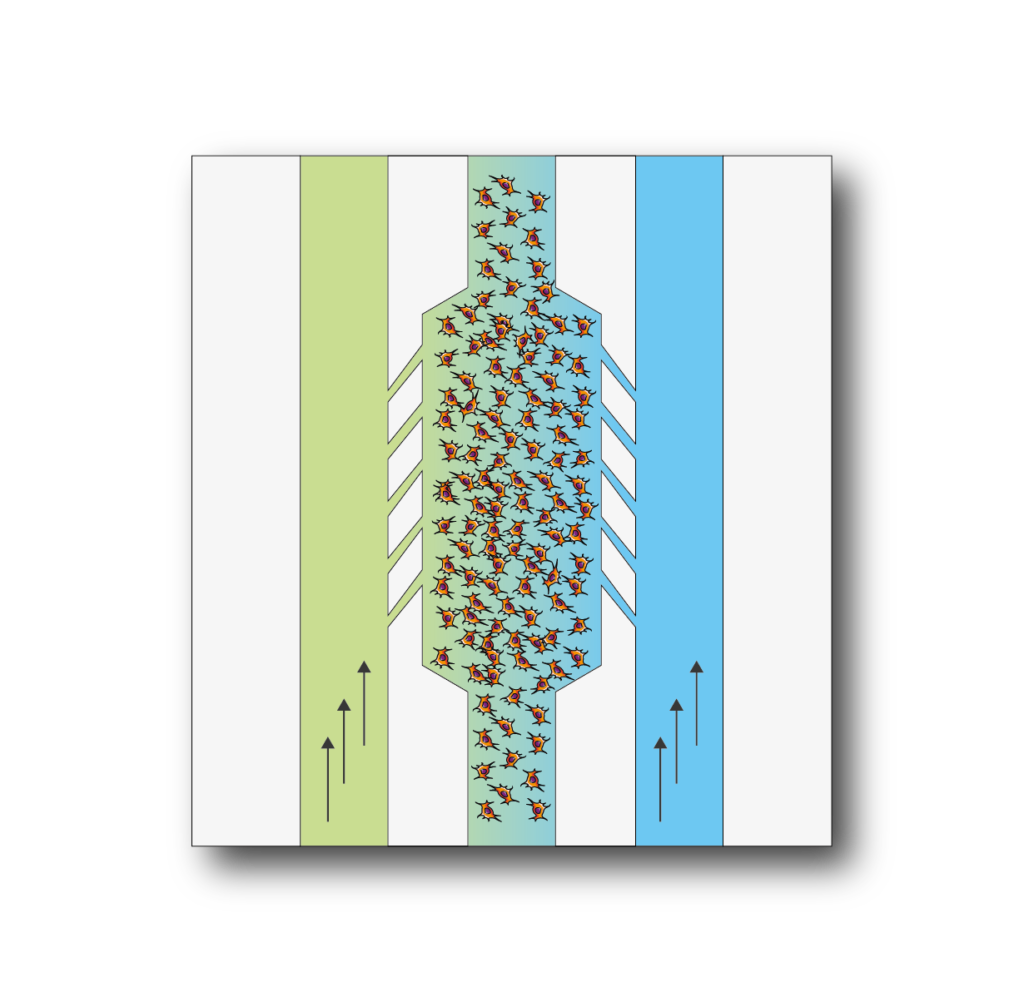
BE-Gradient: Be-Gradient is a device designed to apply electrochemical gradients to 3D cell culture. BE-Gradient is compatible with any type of optical microscope (inverted phase contrast, confocal, fluorescence ......). Be-Gradient consists of a central chamber for cell culture and two transverse channels connected to the central chamber through 3 small microchannels. The Be-Gradient consists of a central chamber for cell culture and two lateral channels connected to the central chamber through 3 small microchannels. The lateral channels are designed to mimic blood vessels. 2D culture can be used not only for walled cells in the central chamber but also for walled cells in the side channels.
II. Cultural patterns
Apply electrochemical gradients to your 3D cell culture.First mix your cells in a liquid hydrogel and then seed them into the central chamber.After polymerization of the hydrogel was completed, culture media with different concentrations of compounds were infused through the lateral channels and the effect was monitored in real time.
III. Application
BE-Gradient is a chip designed with very specific features to study cultures under electrochemical gradients.Things.The device allows for experiments that could never be performed in a petri dish, such as the application of nutrient, oxygen or drug gradients, the study of cell migration under these conditions, angiogenesis studies and more.
Example Application
Trend Migration Study: The video shows a multicellular sphere embedded in collagen and introduced into the microchamber. A fetal bovine serum (FBS) gradient was established across the central chamber by adding serum-containing growth medium to one of the lateral channels. We observed a collective invasion of spheres of oral squamous carcinoma cell OSC-19 in the direction of the FBS gradient. This invasion was more directional and aggressive than the single cells observed in the same experimental setup. Compared to spheres of OSC-19, U87-MG multicellular spheres migrated as single cells. Studies of spheres exposed to chemoattractants showed that diffusion into the spheres was slow, and therefore, chemoattractive waves engulfed the spheres before diffusing through them. The ability to control the trending gradient across the microchamber, coupled with the ability to observe and closely monitor the system over time, makes the BE-gradient a powerful technique for studying the trending process. In recent years, mass invasion has been recognized as a major mode of migration in the development of epithelial tumors. Using BE-Gradient it is possible to observe and analyze this process in vitro.
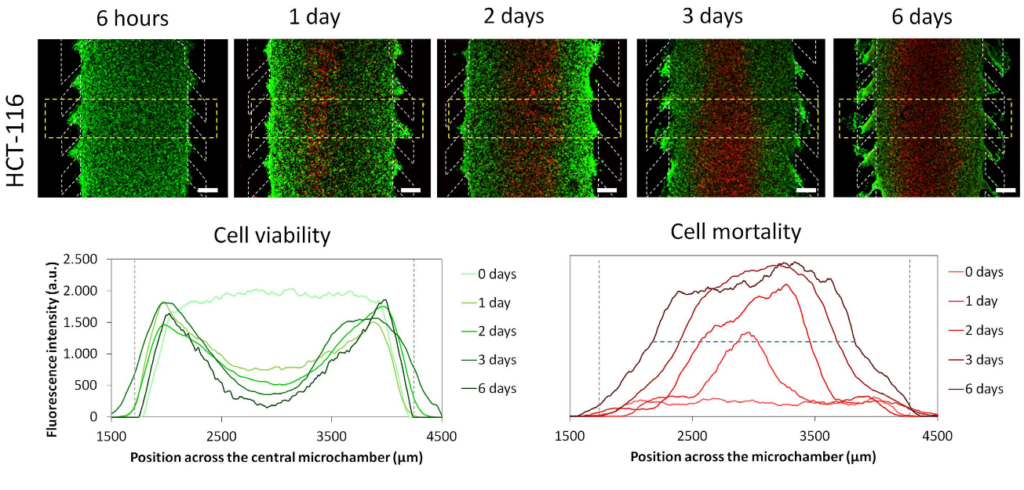
The necrotic core inside the microdevice is generated: HCT-116 cells were embedded in a collagen hydrogel in the central microchamber. 40 million HCT-116 cells/ml were confined in the central microchamber and cell viability was assessed at the indicated times by staining live cells green with calcein (CAM) and dead cells red with propidium iodide (PI). The graph shows the distribution of CAM or PI fluorescence intensity along the area delineated in the image. The positions of the columns are delineated by the gray dotted lines. The width of the necrotic core after 6 days was measured as the distance between those positions in the microchamber that reached a maximum PI fluorescence intensity of 50% (blue dashed horizontal line). The width of the necrotic core was 1643 ± 9 μM, p-value < 0.05. Scale bar was 400 μm.
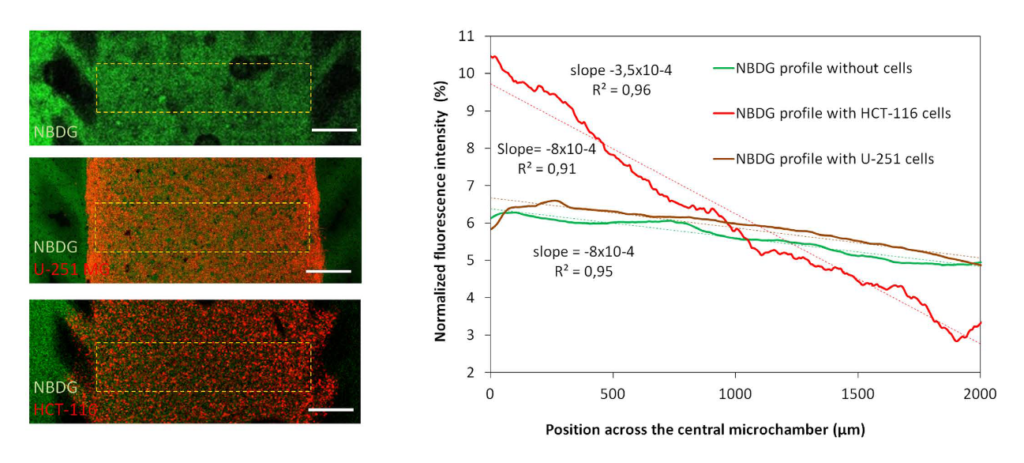
glucose gradient: A green fluorescent glucose analog (NBDG, 200 μ M) was perfused through the left microchannel and the spreading curves were studied in the absence or presence of cells. The graph shows the diffusion curve of NBDG through the central microchamber after 90 minutes, indicating that NBDG can penetrate the collagen hydrogel. The slope of the spreading curves was calculated in the absence or presence of HCT-116 or U-251 MG cells. Scale bar is 400 μm.
Equipment Features
- Easy to use: Be-Gradient is compatible with any type of optical microscope (confocal, fluorescent ......) and has chosen its slide format for easy handling under the microscope.
- Easy to implement: The Be gradient chamber is located in a standard position in the 96-well plate for automated microscopy.
- Easy to connect: Be-Gradient is compatible with all microfluidic flow control systems (syringes, peristaltic pumps, pressure control systems, swing arm systems ......).
- No non-specific absorption: Unlike other PDMS devices, Be-Gradientis is made of a lipophobic thermoplastic material that does not suffer from non-specific drug absorption. Therefore, it allows immunohistochemistry to be performed using fluorescence detection.
- Cell recycling option: Cell cultures used in the Be-Gradient can be easily recycled for further experiments.