I. Client Profile
The client is a key player in Taiwan's pharmaceutical industry, with over 40 years of experience in the market, a mature R&D team and large-scale mass production capabilities. All of its production facilities have passed strict quality certifications such as PIC/S GMP, and its products range from prescription drugs to functional health food and medical consumables.With its high insistence on quality, its brand is trusted both domestically and internationally, and it can be considered as an invisible champion of Taiwan's pharmaceutical industry.
Challenges & Requirements
along withInternational regulations (GDP/GMP) have escalated environmental monitoring requirements.In addition to plant expansion and digital transformation needs, customers face the following core challenges:
1. 24/7 monitoring and compliance pressure
Pharmaceutical production and storage are extremely sensitive to the environment, and the temperature and humidity environment will directly affect the quality of pharmaceutical products, so it is necessary to ensure 24/7 continuous monitoring to comply with the audit specification.
2. Stringent requirements for data landing and information security
3. Rising demand for real-time alerts and centralized management
The old system was fragmented, had inconsistent interfaces, and lacked real-time and integration capabilities. The client needed a system that could centrally manage dozens or hundreds of devices and support LINE and Email alerts.As soon as the temperature and humidity exceeds the standard, the administrator can be notified immediately.
4. Build to avoid interfering with production
Most of the plants are pre-existing buildings that contain clean room areas that do not allow for large scale drilling or physical wiring work.
III. The Solution
After evaluating a number of options, the client finally adoptedHonghong COMET Wi-Fi Temperature and Humidity Monitoring SystemIn addition, the company has successfully met the three requirements of "no wires", "security in place" and "compliance".
⭐ Core Technology Highlights
1|Wi-Fi Wireless Deployment, Plug and Play
Optional COMET Wi-Fi temperature and humidity sensor, using the plant's existing Wi-Fi network for communication. This eliminates the need for traditional RS-485 signal lines and greatly reduces interference with the cleanroom and production line, and requires only power to operate.
2|Modbus TCP serial ground terminal system, zero data outflow


The actual deployment of COMET Wi-Fi temperature and humidity monitoring system inside the plant.
3|Visualized environmental control platform and automatic alarms
4|Output GMP Compliance Report in one click
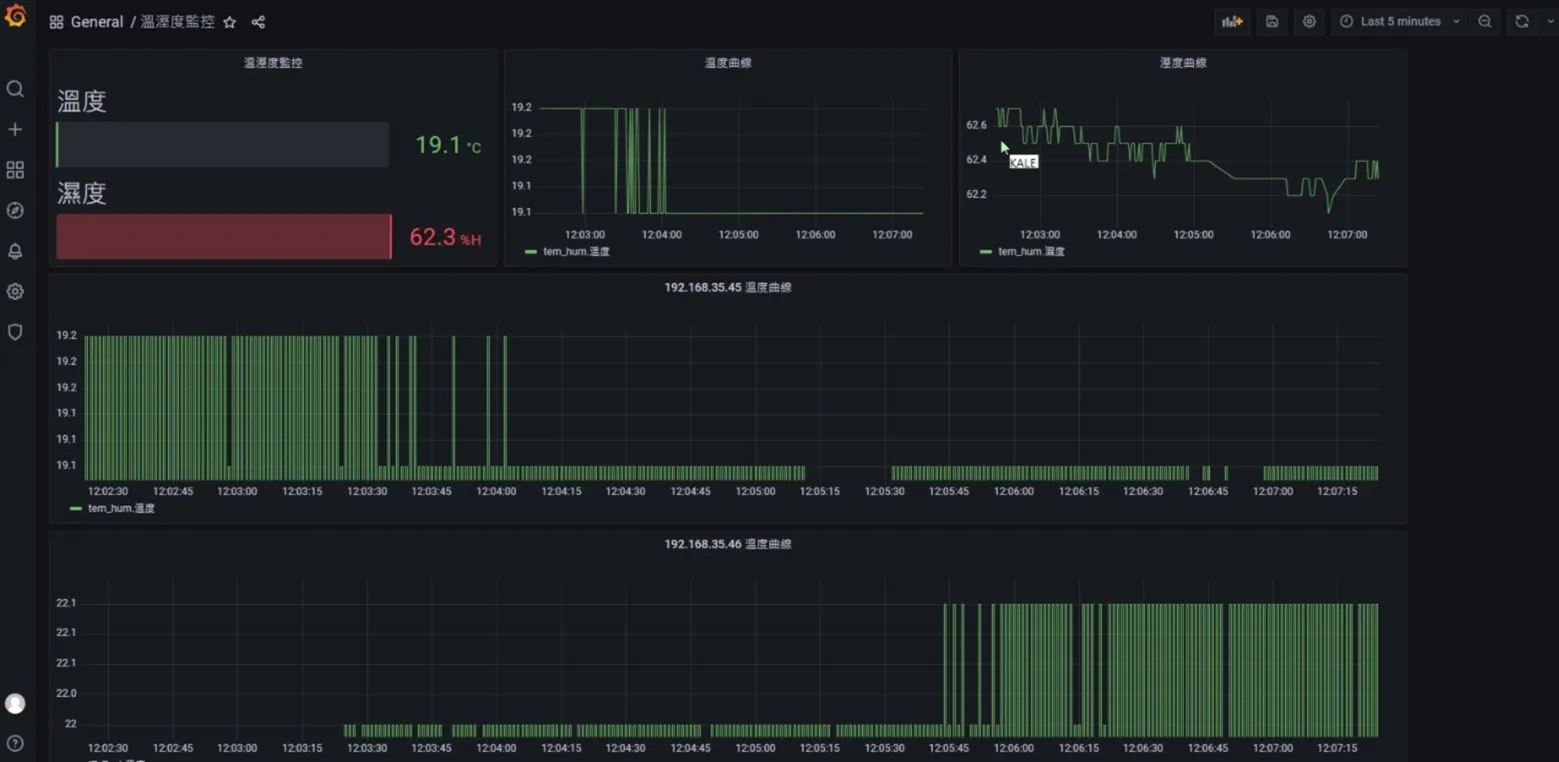
IV. Project Benefits and Conclusion
After the implementation of Honghong COMET, the customer has successfully upgraded the entire factory environment control and gained the following benefits:
1. Compliance Enhancement
Data Integrity makes it easy to pass every audit and ensures consistent quality for every batch of drugs leaving the factory.
2. Significant improvement in management efficiency
Eliminating the need for manual transcription and inspection, automated alarms allow managers to respond to abnormalities immediately and minimize risks.
3. Lighter and more maintenance friendly.
The Wi-Fi architecture dramatically reduces setup time, and the local storage model meets security requirements without the need for expensive cloud subscription fees.
4. Transparency and visibility of cross-departmental information
V. Conclusion: The Next Step to Enhancing Pharmaceutical Competitiveness: Turning Environmental Controls from a Cost to Your Advantage
After choosing Honghong's COMET solution, this pharmaceutical company upgraded their environmental monitoring system andWe have formally mastered a set of long-term competitiveness that can "continuously pass audits, continuously reduce risks, and continuously improve the stability of production lines".
In the highly regulated pharmaceutical industry, a single failure in environmental control is tantamount to scrapping a batch of material, delaying a schedule, or even missing a market.Instead of a simple sensor, COMET provides a set of sensors that can be trusted by QA, IT, and the production line. Compliance Framework:
Zero data outflow, zero monitoring breakpoints, zero wiring interference.
That's why more and more PIC/S GMP factories consider Macronix COMET as the standard equipment "the least worry and the most acceptable to the auditor".In the pharmaceutical industry, the biggest uncertainty is the risk of environmental control; Honghong's professional role is to help companies minimize these uncertainties!This is the core reason why so many customers have chosen and continue to trust Honghong!
✔ GMP/GDP Environmental Upgrade
✔ Intranet (On-Premise) Architecture
✔ Clean room low interference deployment
✔ SQL/SCADA Serialization
✔ Multi-site large-scale monitoring
👉We can help you achieve "one-time planning, long-term peace of mind"! Contact Hong Hong today! Obtain customized project evaluation and architecture deployment planning.Make your environmental control system less about cost pressure and more about industry competitiveness!
Honghong will provide you with any support you need!
Our professional Honghong team will be the first to respond and provide you with the best service to solve all your problems.



